A woman in her 40s was diagnosed with impaired glucose tolerance, or poor blood sugar control, after taking a health product that she obtained from a relative in Malaysia.
The item, Herba Saraf, which was bought online, was labelled to contain herbal ingredients for the relief of pain, such as joint pain and migraine.
The Health Sciences Authority (HSA) said in a statement on Tuesday that the woman had taken it for one month for her knee pain. She stopped taking the product immediately after suspecting that her impaired glucose tolerance was caused by Herba Saraf.
Impaired glucose tolerance is a condition that may increase one’s risk of diabetes and heart disease.
HSA said the product was tested and found to contain dexamethasone, a potent steroid that is a prescription-only medicine that should only be used under medical supervision.

The authority added that inappropriate and prolonged use of steroids can result in Cushing’s syndrome, increased blood glucose levels leading to diabetes, high blood pressure, cataracts, muscular and bone disorders, and an increased risk of infections.
Herba Saraf is just one of three products that HSA tested and found potent undeclared ingredients.
HSA also found high levels of mercury in two other cosmetic sets from Malaysia: ‘Wonderglow’ and ‘Tati Skin Care 5 in 1’.

Both items are being sold online. A woman was detained at the Causeway on Dec 2 last year while attempting to bring the cosmetic sets into Singapore.
‘Wonderglow’ was marketed as an anti-wrinkle and anti-ageing product, with claims to brighten the skin in “as early as three days”. It was also falsely labelled as “100 per cent No Mercury Guaranteed”.
However, HSA found that the night cream in the Wonderglow set contained very high levels of mercury, exceeding permissible limits by 7,000 times.


HSA had alerted the public to stop buying and using the ‘Tati Skin Care 5 in 1’ in June last year, but the product has resurfaced online again.
Tests showed that the ‘Therapy Cream 1’ in the set contained mercury exceeding the permissible limits by close to 50,000 times.
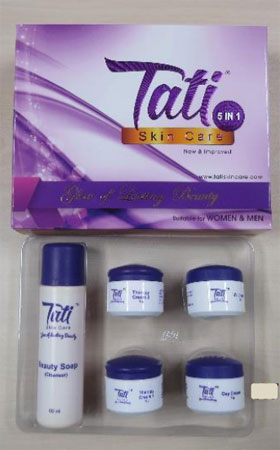
‘Therapy Cream 2’ in the set was tested to contain hydroquinone and tretinoin. Both are potent western ingredients that should only be used under medical supervision.
HSA said long-term exposure to cosmetic products with high levels of mercury can cause serious health consequences, including damage to the kidneys, and digestive and nervous systems. Inappropriate use of hydroquinone and tretinoin could cause adverse skin reactions such as redness, burning and peeling of the skin.
HSA advises consumers to stop using all three products and to see a doctor as soon as possible if you have taken Herba Saraf.
Consumers should also be cautious when purchasing health products online or from unfamiliar sources, even if they are recommended by friends or relatives, advised HSA.
HSA also issued a warning to sellers and suppliers of the illegal health products.
Anyone who supplies illegal health products is liable to prosecution and if convicted, may be imprisoned for up to three years and fined up to $100,000.
klim@sph.com.sg

More about
skincare HSA (Health Sciences Authority) illegal





