SINGAPORE: Three brands of high blood pressure medicine containing the ingredient losartan have been recalled because they contain higher than acceptable levels of a potentially harmful impurity, the Health Sciences Authority (HSA) said on Thursday (Mar 28).
The drugs contain the active ingredient losartan potassium, which was manufactured by Indian pharmaceutical company Hetero Labs.
The affected products are the 50mg and 100mg tablets from the brands Losartas, Losagen and Hyperten and distributed by local suppliers Apotheca Marketing, Medicell Pharmaceutical and Goldplus Universal, respectively.
About 137,000 patients in Singapore are using the three recalled brands of losartan medicine, said the Ministry of Health (MOH).
Losartas is prescribed in public healthcare institutions, while Losartas, Losagen and Hyperten are prescribed at private healthcare institutions.
HSA has advised patients not to stop treatment on their own as there is no immediate health risk and sudden stopping of the drugs can pose greater immediate risk to their health.
READ: 137,000 patients affected by losartan high blood pressure medicine recall: MOH

(Source: Health Sciences Authority)
HIGH EXPOSURE OVER A LONG PERIOD MAY INCREASE CANCER RISK
The recalled products were found to contain trace amounts of a nitrosamine impurity, N-nitro-N-methyl-4-aminobutyric acid (NMBA), which are above internationally accepted levels, HSA said.
Exposure to nitrosamines at high quantities over a long-term period may potentially increase the risk of cancer.
For example, the added cancer risk from an additional six-month exposure is estimated to be less than 0.0002 per cent.
“The risks of trace amounts of NMBA are associated with long term exposure. Sudden stopping of the medicines can pose greater and more immediate risk to patient’s health … We have advised healthcare professionals to review the medicine and treatment plans of their patients,” HSA said.
The affected products contain the active ingredient losartan potassium. (Photo: Health Sciences Authority)
Affected consumers are advised to consult their medical providers. (Photo: Health Sciences Authority)
HSA said that several losartan medicines have been recalled overseas since end-February due to the presence of NMBA. It tested all locally marketed losartan products for the presence of the NMBA impurity and in March found the three brands contained trace amounts of NMBA above acceptable levels.
The other seven brands of losartan medicines marketed in Singapore are not affected by this impurity.
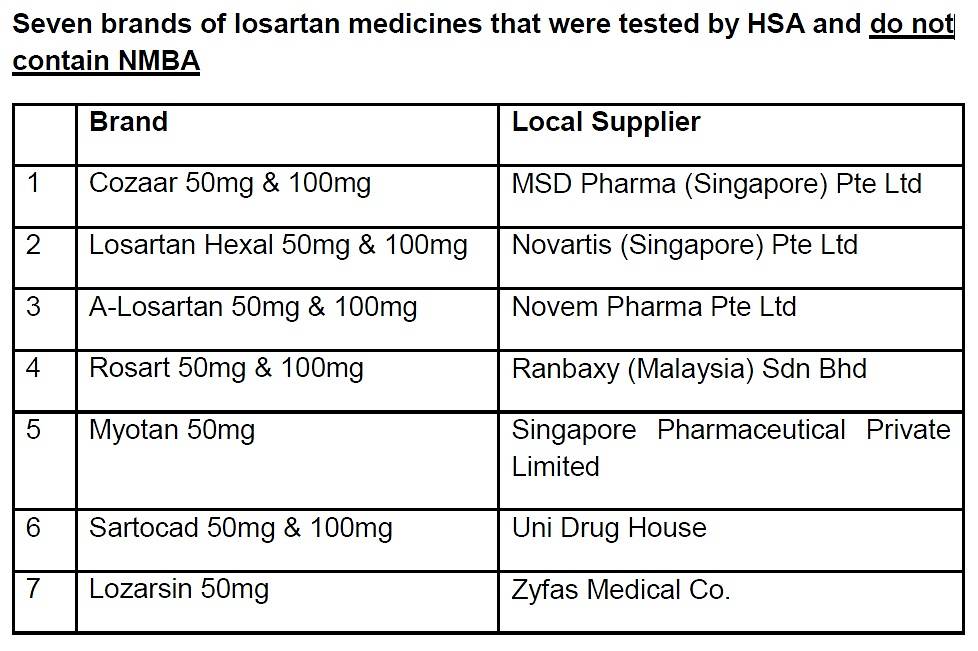
(Source: Health Sciences Authority)
NO IMMEDIATE HEALTH RISK
National Heart Centre Singapore Cardiologist Professor Ding Zee Pin said that there is no immediate health risk associated with taking the affected medicines and that patients are advised not to stop or change treatment on their own.
“As losartan is used to treat high blood pressure, stopping the medicine without replacement of other equivalent medication can increase the risk of poor control of blood pressure,” said Professor Ding, who is also on HSA’s Expert Panel on Nitrosamines.
Consumers who are taking the affected medication are advised to discuss their treatment plan with their healthcare provider.
“Do not stop taking the medicines on your own until you have been provided with a replacement brand of losartan or a different medicine by your healthcare provider,” HSA said.
MOH said in its release that public healthcare institutions will be reaching out to their patients.
Those with medical appointments scheduled before Jul 1 should proceed with their appointments, at which point their doctor will advise them on alternative medicines, MOH said.
Patients whose medical appointments are scheduled on or after Jul 1 will be contacted for an earlier consultation.
TESTS CONDUCTED ON OTHER BLOOD PRESSURE MEDICATION
Losartan belongs to a class of medicines called angiotensin II receptor blockers (ARBs), which are used to treat high blood pressure, also known as hypertension.
HSA said that since June 2018, several ARB medicines have been recalled overseas due to the presence of two other nitrosamine impurities, N-Nitrosodimethylamine (NDMA) and N-Nitrosodiethylamine (NDEA).
HSA said it has tested the locally marketed ARB medicines and found that none of them contained unacceptable levels of the two impurities.
“HSA is working with companies and international regulatory agencies to verify the cause of contamination, and to formulate measures to address the issue,” it said.
“HSA will require companies to make the necessary changes to their manufacturing process to ensure that the medicines do not contain these impurities in future,” it added.





