SINGAPORE: The Health Sciences Authority (HSA) issued an alert on Thursday (Dec 22) on three health products which have caused serious adverse reactions, and in one case, resulted in a woman being hospitalised. The products were found to contain undeclared potent substances, including sibutramine, which is banned.
One of the products is called Ananda Thukha Remedy for Diabetes. A woman in her 60s took it and ended up with agranulocytosis, a serious blood disorder in which a patient has a low white blood cell count and is prone to falling ill from infections. She was hospitalised for almost two weeks.
The woman had bought the product from a shop in Peninsula Plaza after her friend recommended it. HSA has seized the product from the retail store for further investigations.

Ananda Thukha Remedy for Diabetes. (Photo: HSA)
Another product called 1 Day Diet was touted as a “100% pure natural” slimming product with “quick effect” and “no side effect”.
However, a healthy woman in her 30s who purchased the product online had experienced breathing difficulties, an increased heart rate and excessive sweating of palms after taking the capsules for less than two weeks.
HSA said this was due to the ingredient Sibutramine, which has been disallowed for sale in Singapore since 2010 due to “serious safety concerns”. The use of sibutramine may cause serious adverse effects like high blood pressure, irregular heartbeats, hallucinations and mood swings.
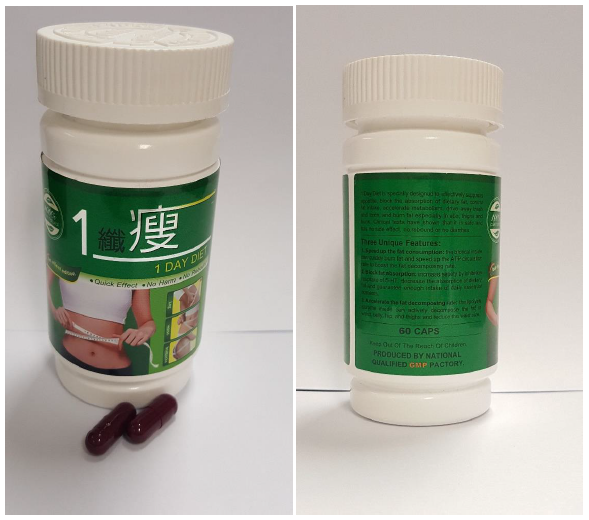
1 Day Diet. (Photo: HSA)
HSA is also warning members of the public against Bee Brand Qi Li Xiang, supposedly for body aches. But a man in his 50s, who took the product for about a month, ended up with low blood pressure and low levels of the hormone cortisol – symptoms typically associated with steroid consumption, and if left untreated, could lead to serious health complications.
HSA added that Bee Brand Qi Li Xiang contained Dexamethasone, which could cause high blood pressure, cataracts, increase risk of infections and Cushing’s syndrome if taken long-term without medical supervision.

Bee Brand Qi Li Xiang’s inner packaging. (Photo: HSA)
Associate Prof Chan Cheng Leng, acting group director of the Health Products Regulation Group at HSA said: “Consumers need to be wary of complementary health products that offer quick cures and relief of chronic illnesses. Do not be enticed by their false promises as they may contain undeclared potent ingredients that can pose serious health risks.”
The authority added that members of the public should exercise caution when purchasing health products online, or from other unfamiliar sources, even if it’s from friends of family who meant well.
As for retailers, those caught selling illegal health products could be prosecuted under the Health Products Act, Poisons Act and/or Medicines Act. Under the Health Products Act, for instance, offenders could face a fine of up to S$100,000, up to three years’ jail or both.
HSA also urged members of the public who have information on the sale or supply of illegal products to contact its enforcement branch at 6866-3485, or email hsa_is@hsa.gov.sg.




